STEP 2: Break into its component irreducible representations. STEP 3: Subtract rotations and translations to find vibrational modes. STEP 4: Determine which of the vibrational modes are IR-active and Raman-active. Infrared selection rules: Raman selection rules: Summary of Analysis for Water. Acknowledgements.
Origin of Rate and Selectivity Trends and Exceptional Yield in Sulfur-Oxidative Propane Dehydrogenation Over Supported Vanadium Catalysts | ACS Catalysis
Homonuclear diatomic molecules= no electric dipole moment = no change in μ during the vibration = do not show IR absorption-Heteronuclear diatomic molecules= have a dipole moment, that changes during the vibration = can be studied by IR absorption spectroscopy-Polyatomic molecules with several different vibrational modes, sometimes all of these are IR active; sometimes some vibrations do
![Solved [The numbers of IR active & Raman active] Please | Chegg.com](https://media.cheggcdn.com/media%2Fb0a%2Fb0a7ffce-6c5f-4a68-a16f-19078f0f4200%2Fphpl9oaWI.png)
Source Image: chegg.com
Download Image
Science Chemistry Chemistry questions and answers Select the vibrations that should be infrared active. options (CH3CH2)2C=O , the C=O stretch (CH3CH2)3C−Cl , the C−Cl stretch CH3CH2C≡CCH2CH3 , the C≡C stretch CH3CH2CH2C≡CH , the C≡C stretch trans‑4‑octene, the C=C stretch This problem has been solved!

Source Image: mdpi.com
Download Image
Fuels | Free Full-Text | Calibration Method for the Determination of the FAME and HVO Contents in Fossil Diesel Blends Using NIR Spectroscopy Dec 6, 2022For example, the C=C stretch found in CH; CHCHC_CH and trans-4-octene, is a vibrational mode that is often infrared active. This is because the vibration changes the dipole moment of the molecule, a necessary condition for infrared activity. Similarly, the C=0 stretch in CH;C CH2h ‘ C=O and the €-Cl stretch in (CH3)3C-C1, alter the dipole

Source Image: image-sensors-world.blogspot.com
Download Image
Select The Vibrations That Should Be Infrared Active
Dec 6, 2022For example, the C=C stretch found in CH; CHCHC_CH and trans-4-octene, is a vibrational mode that is often infrared active. This is because the vibration changes the dipole moment of the molecule, a necessary condition for infrared activity. Similarly, the C=0 stretch in CH;C CH2h ‘ C=O and the €-Cl stretch in (CH3)3C-C1, alter the dipole 12.3: IRActive and IR-Inactive Vibrations. Some bonds absorb infrared light more strongly than others, and some bonds do not absorb at all. In order for a vibrational mode to absorb infrared light, it must result in a periodic change in the dipole moment of the molecule. Such vibrations are said to be infrared active.
Image Sensors World: October 2013
11.3: IRActive and IR-Inactive Vibrations Asymmetry and polarity increase the strength of IR absorption (infrared active). Symmetrical carbon-carbon double and triple bonds will not absorb IR light and are called “infrared … Infrared spectroscopy(IR) & FTIR (Analytical Technique) | PPT

Source Image: slideshare.net
Download Image
Principles of infrared spectroscopy (1) Molecular vibrations and infrared absorption | JASCO Global 11.3: IRActive and IR-Inactive Vibrations Asymmetry and polarity increase the strength of IR absorption (infrared active). Symmetrical carbon-carbon double and triple bonds will not absorb IR light and are called “infrared …
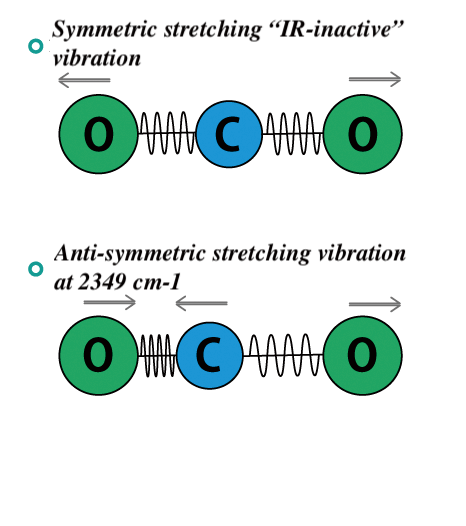
Source Image: jasco-global.com
Download Image
Origin of Rate and Selectivity Trends and Exceptional Yield in Sulfur-Oxidative Propane Dehydrogenation Over Supported Vanadium Catalysts | ACS Catalysis STEP 2: Break into its component irreducible representations. STEP 3: Subtract rotations and translations to find vibrational modes. STEP 4: Determine which of the vibrational modes are IR-active and Raman-active. Infrared selection rules: Raman selection rules: Summary of Analysis for Water. Acknowledgements.

Source Image: pubs.acs.org
Download Image
Fuels | Free Full-Text | Calibration Method for the Determination of the FAME and HVO Contents in Fossil Diesel Blends Using NIR Spectroscopy Science Chemistry Chemistry questions and answers Select the vibrations that should be infrared active. options (CH3CH2)2C=O , the C=O stretch (CH3CH2)3C−Cl , the C−Cl stretch CH3CH2C≡CCH2CH3 , the C≡C stretch CH3CH2CH2C≡CH , the C≡C stretch trans‑4‑octene, the C=C stretch This problem has been solved!
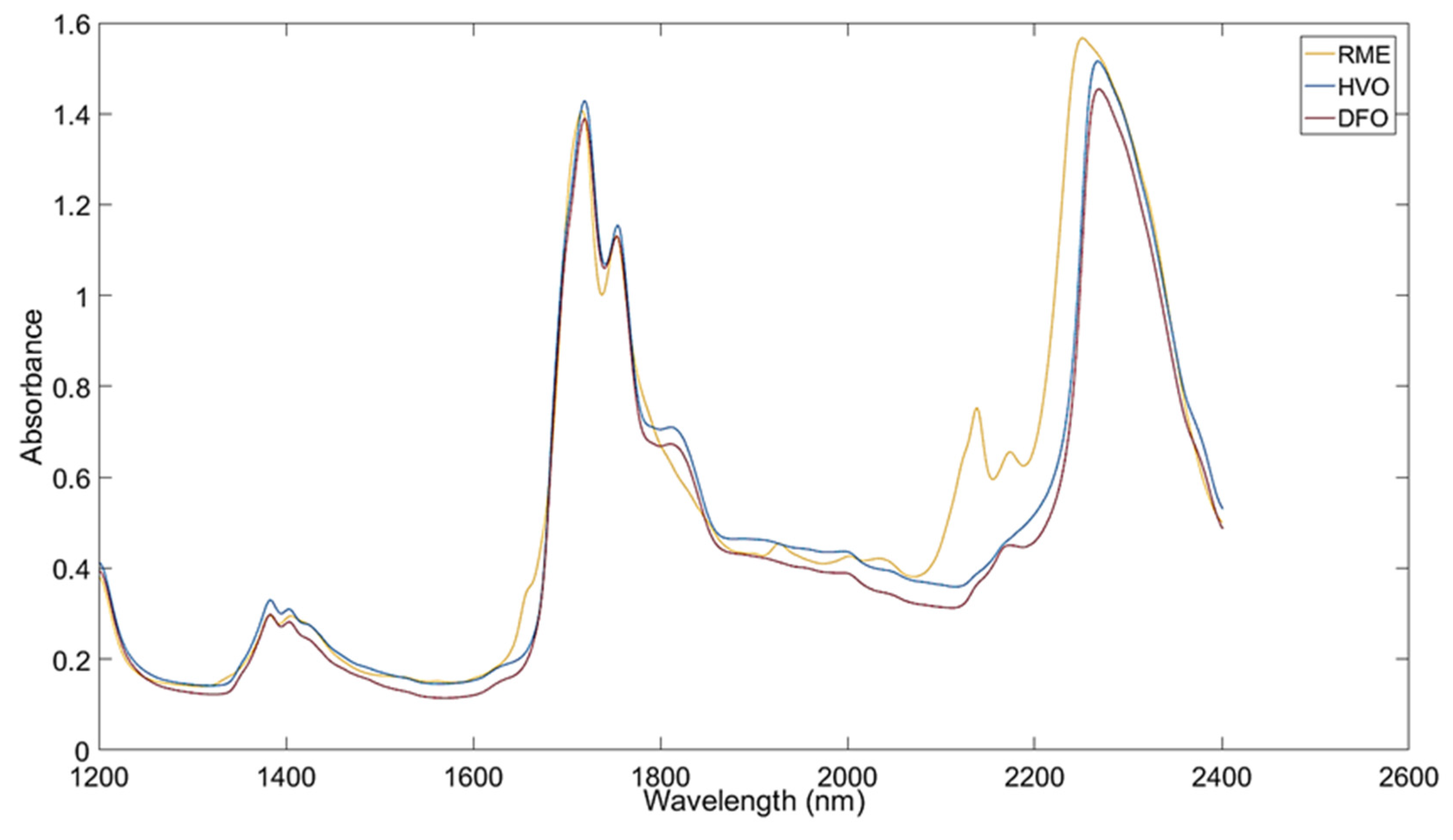
Source Image: mdpi.com
Download Image
Solved Select the vibrations that should be infrared active. | Chegg.com Step 1 1 of 8 In this problem, we have to explain which of the given vibrations should be infrared-active and which should be infrared-inactive. First, we have to know that infrared-activeare those vibrations that result in a periodic change in the dipole moment of the molecule.
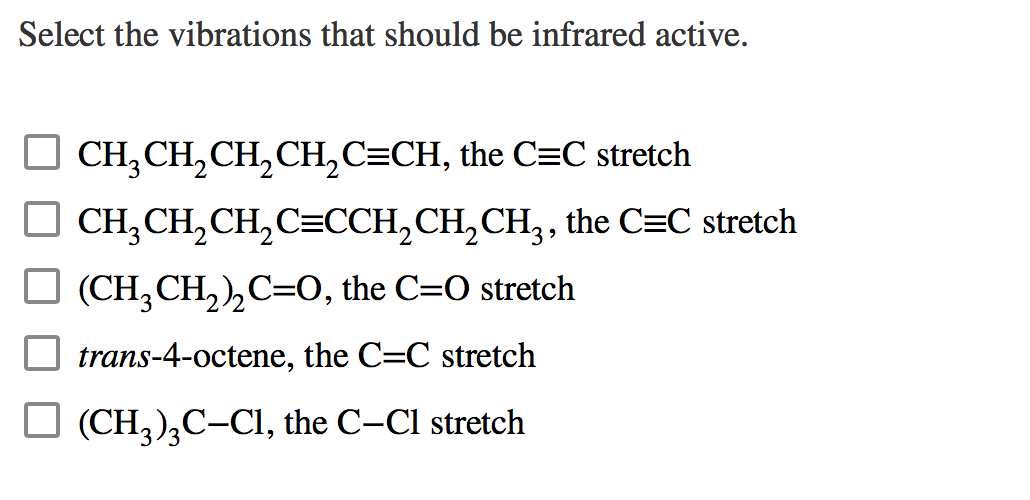
Source Image: chegg.com
Download Image
ORGANIC SPECTROSCOPY INTERNATIONAL: IR SPECTROSCOPY Dec 6, 2022For example, the C=C stretch found in CH; CHCHC_CH and trans-4-octene, is a vibrational mode that is often infrared active. This is because the vibration changes the dipole moment of the molecule, a necessary condition for infrared activity. Similarly, the C=0 stretch in CH;C CH2h ‘ C=O and the €-Cl stretch in (CH3)3C-C1, alter the dipole
Source Image: orgspectroscopyint.blogspot.com
Download Image
Tb- and Eu-doped yttrium oxyselenides as novel absorber layers for superstrate thin-film photovoltaics: improved spectral optical absorption and green … – Nanoscale (RSC Publishing) DOI:10.1039/D3NR01162C 12.3: IRActive and IR-Inactive Vibrations. Some bonds absorb infrared light more strongly than others, and some bonds do not absorb at all. In order for a vibrational mode to absorb infrared light, it must result in a periodic change in the dipole moment of the molecule. Such vibrations are said to be infrared active.
Source Image: pubs.rsc.org
Download Image
Principles of infrared spectroscopy (1) Molecular vibrations and infrared absorption | JASCO Global
Tb- and Eu-doped yttrium oxyselenides as novel absorber layers for superstrate thin-film photovoltaics: improved spectral optical absorption and green … – Nanoscale (RSC Publishing) DOI:10.1039/D3NR01162C Homonuclear diatomic molecules= no electric dipole moment = no change in μ during the vibration = do not show IR absorption-Heteronuclear diatomic molecules= have a dipole moment, that changes during the vibration = can be studied by IR absorption spectroscopy-Polyatomic molecules with several different vibrational modes, sometimes all of these are IR active; sometimes some vibrations do
Fuels | Free Full-Text | Calibration Method for the Determination of the FAME and HVO Contents in Fossil Diesel Blends Using NIR Spectroscopy ORGANIC SPECTROSCOPY INTERNATIONAL: IR SPECTROSCOPY Step 1 1 of 8 In this problem, we have to explain which of the given vibrations should be infrared-active and which should be infrared-inactive. First, we have to know that infrared-activeare those vibrations that result in a periodic change in the dipole moment of the molecule.